Agilis Presents Results for Angelman Syndrome Gene Therapy at ESGCT, NORD
Written by |
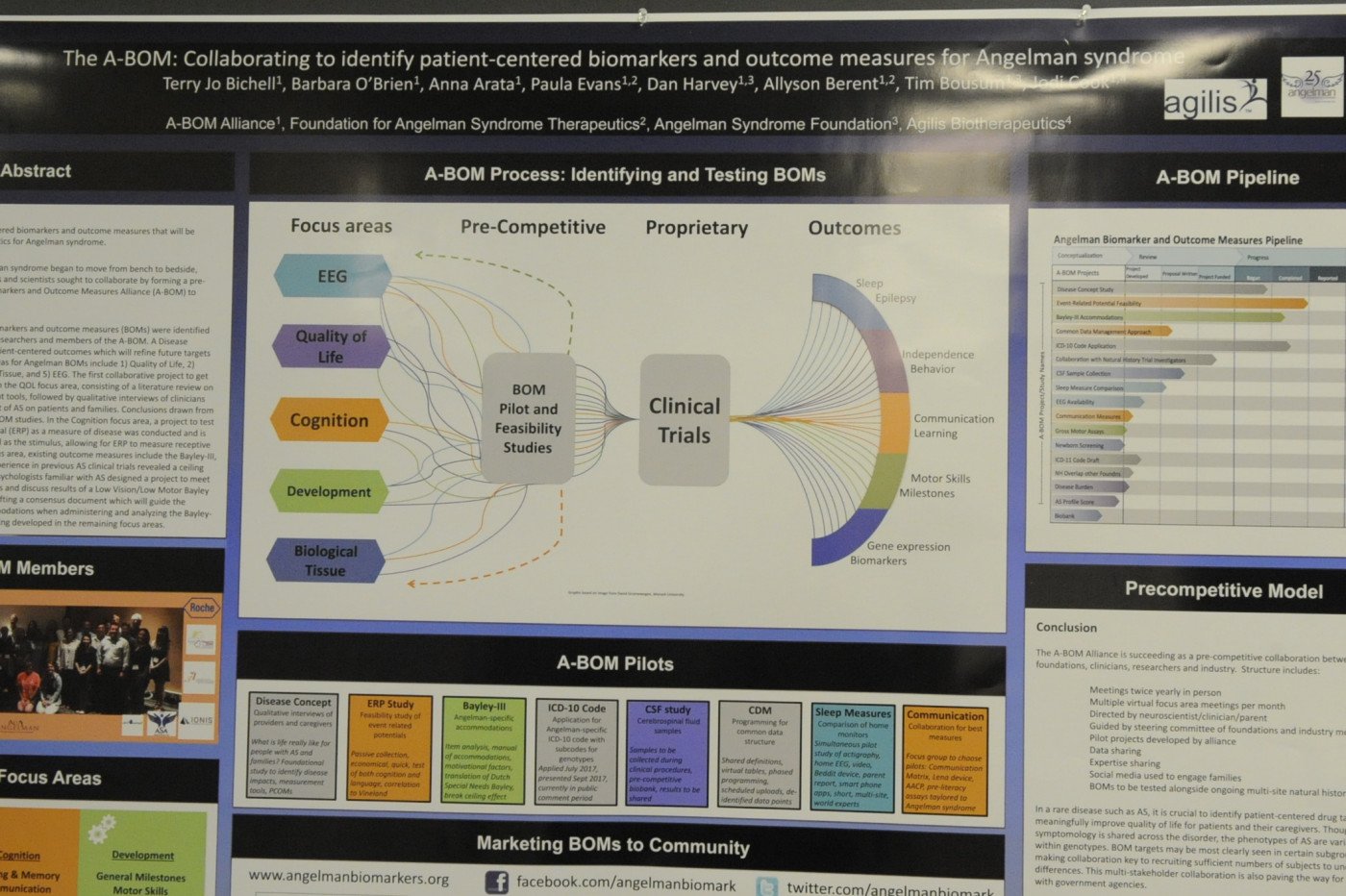
Dr. Terry Jo Bichell's poster presentation on Angelman syndrome.
Agilis Biotherapeutics, which develops innovative DNA therapeutics for rare diseases of the central nervous system, presented the latest results of its gene therapy program for Angelman syndrome at two recent international conferences.
During the annual meeting of the European Society of Gene and Cell Therapy (ESGCT), Dr. Kevin Nash, an Agilis researcher at the University of South Florida (USF), presented the poster “Expression of Human UBE3A via AAV5 Rescues Spatial Learning and Synaptic Plasticity Deficits in a Model of Angelman Syndrome.”
The data from in vivo studies uses an established mouse model for Angelman syndrome. The Agilis gene therapy – an adeno-associated virus (AAV) vector with the human UBE3A gene – was delivered directly into the central nervous system of mice. USF researchers confirmed that UBE3A was reached and was successfully expressed throughout the central nervous system.
Researchers also tested the therapy’s effects on the activity of a brain region called the hippocampus, which is vital for learning and memory. That restorative effect improved certain functional abilities affected in Angelman mice models and in Angelman patients, including spatial learning in a water maze test.
Overall, the therapy restored synaptic activity in the hippocampus and promoted learning and formation of new memories in adult mice with Angelman syndrome.
“The presentation of our new data on Angelman syndrome gene therapy at ESGCT continues to validate Agilis’ approach to development of our gene therapy program for Angelman syndrome,” Dr. Jodi Cook, chief operating officer for Agilis, said in a press release.
These results extend on Agilis previous findings and show for the first time that intracerebroventricular delivery (directly into the brain) of the human UBE3A gene can rescue key functions in an established Angelman syndrome mouse model. They also support Agilis’ brain-targeted AAV therapy potential for clinical trials.
A second poster, “The A-BOM: Collaborating to identify patient-centered biomarkers and outcome measures for Angelman Syndrome,” was presented at the National Organization for Rare Diseases (NORD) Rare Disease Summit by Dr. Terry Jo Bichell, scientific officer at the Angelman Syndrome Biomarker and Outcome Measures (A-BOM) Alliance.
Agilis is a founding member of the A-BOM Alliance, which promotes research in Angelman syndrome to identify potential disease biomarkers that will help researchers evaluate the efficacy of therapeutics in future clinical trials. The alliance has established five focus areas: quality of life (including sleep); cognition (including language); development (including motor skills and the use of validated scales); biological tissues (including plasma and cerebral spinal fluid measures); and seizures (EEG measurements).
A-BOM hope to identify future areas of research and promote sharing of this data within its members at annual meetings.
“We were delighted to share our experience with other rare disease groups at the NORD conference,” said Bichell. “We have a great model of pre-competitive cooperation that was initiated by Agilis Biotherapeutics, and because of this, progress has accelerated to finding treatments for Angelman syndrome.”
Added Cook: “As a pioneer industry partner with the Angelman community, we are thrilled to see the important and material advances being made in fundamental areas in the understanding of AS that are a result of the A-BOM alliance.”





